2015
Quanterix Names PBL Assay Science as a Preferred Partner in Assay Development and Testing Services
In response to growing customer demand, PBL will leverage Quanterix' Simoa technology to provide enhanced sensitivity for assay development across a broad range of therapeutic areas.
Lexington, MA, June 15, 2015 -- Quanterix Corporation, a leader in high definition diagnostics, delivering ultrasensitive single molecule measurement for the benefit of human health, today announced an alliance with PBL Assay Science (PBL), naming the Company as a Preferred Partner for assay research and development. Leveraging Simoa’s sensitivity and revolutionary digital approach for assay development and research, PBL will have the ability to enter new markets and strengthen its current offerings.
As a leading provider of products, services, information, and know-how related to interferons, cytokines, antibodies, ELISAs, and assay solutions, PBL Assay Science is working hand in hand with industry, academic and government researchers to solve difficult assay development and protein quantification problems for its customers. Using Simoa’s single molecule detection capabilities, PBL will be able to provide their customers with a completely automated experience, ensuring consistent results and the greatest sensitivity possible.
“PBL’s alliance with Quanterix is a natural fit for our companies. Incorporating Simoa’s ultrasensitive single molecule measurement into our pipeline of assay development and services capabilities allows us to enter new markets and significantly strengthen current offerings,” said Dr. Thomas Lavoie, Chief Scientific Officer, PBL. “We have built a robust line of ELISA, protein, and antibody products, and have extensive experience in developing and executing high sensitivity biomarker assays. These capabilities are well complemented by Quanterix’ Single Molecule Array technology to provide answers to problems in disease biology where basal levels of biomarker analytes have been extremely difficult to quantify. We anticipate our collaboration with Quanterix will bring unique content onto the platform and deliver high sensitivity assay development.”
“We couldn’t be happier to add PBL to our preferred partner program,” said Kevin Hrusovsky, CEO and Executive Chairman at Quanterix. “By combining their deep-rooted expertise in solving difficult assay development and protein quantification problems with the service offerings available in our own Simoa HD-1 Accelerator Lab, we’re confident that this partnership will accelerate assay development and lead to groundbreaking discoveries in diverse therapeutic areas of research.”
To learn more about Simoa, please visit: www.quanterix.com.
About PBL
PBL Assay Science is a major provider of products, services, information, and know-how related to interferons, cytokines, antibodies, ELISAs, and assay solutions. The company employs state-of-the-art technology in conducting assay services and manufacturing world-class products for scientists around the globe each day. Learn more at https://www.pblassaysci.com.
About Quanterix
Quanterix is a developer of ground-breaking tools in high definition diagnostics. Its Simoa platform uses single molecule measurements to access previously undetectable proteins. With this unprecedented sensitivity and full automation, Simoa offers significant benefits to both research and clinical testing applications. Quanterix was established in 2007 and is located in Lexington, Massachusetts. To learn more about Quanterix and Simoa, please visit: www.quanterix.com.
2014
PBL Assay Science broadens offerings with authentic human cell-expressed cytokines and growth factors in licensing agreement with HumanZyme, Inc.
PISCATAWAY, N.J., Feb. 19, 2014 /PRNewswire/ -- authentic human cell-expressed (HCE) cytokines and growth factors directly to scientists worldwide.
HumanZyme, Inc. is a global leader in providing highly authentic recombinant proteins from human cells under the HumanKine® brand and a preferred outsourcing supplier of human protein production. These human cell-expressed proteins mimic native human proteins more closely than proteins from non-human expression systems, such as E. coli, in terms of glycosylation, assembly, location, and structure.
PBL Assay Science is a major provider of products, assay services, and information related to interferons, cytokines, antibodies, ELISAs, and assay science. The company employs state-of-the-art technology and years of know-how in conducting assay services and manufacturing world-class products for scientists around the globe.
"HumanZyme's leadership and innovations in authentic human proteins provide a perfect complement to our unique cytokine and interferon reagents, ELISA, and assay service offerings," said Robert Pestka, CEO, PBL Assay Science. "Introducing these proteins to our product line enhances our ability to help scientists approach their experiments using more biologically relevant research tools."
"For over twenty years, PBL has grown not only in number of products offered but in knowledge around interferons, cytokines and assay science," said Sean Doctor, Executive Vice President and GM, HumanZyme, Inc. "Combining our human cell-expressed reagents with PBL's expertise expands both companies' capability to address researchers' most difficult problems."
The agreement between PBL Assay Science and HumanZyme, Inc. begins this month. Human cell-expressed (HCE) proteins in the agreement include members of the human TGF beta, Fibroblast Growth Factor (FGF), Hematopoietin and Interleukin, and Tyrosine Kinase Receptor Signaling families, and TNF-alpha. To learn more, contact PBL Assay Science.
2013
PBL InterferonSource changes name to PBL Assay Science reflecting broader offerings
September 18, 2013, Piscataway, NJ, USA/PRNewswire/ --- PBL InterferonSource, a major provider of products, services, information, and know-how related to interferons, cytokines, antibodies, ELISAs, and assay services is changing its name to PBL Assay Science. The company has already begun to implement these changes.
The name change reflects the company's growth beyond interferons to a full range of assay-related products and services. For over two decades PBL, based on Piscataway, NJ, has grown its reputation as a trusted partner by working hand in hand with researchers to help solve their most difficult problems.
"Everything we do is focused one way or another on assays. As we see it, PBL has three basic areas of offerings: Proteins, ELISAs, and Services. We will maintain these three focus areas and work within each to broaden assay offerings to our customers," said Robert Pestka, CEO of PBL Assay Science. "In doing this we have not abandoned our core competency in interferons: we have simply recognized that our research, our products, and our services have moved beyond interferon into other cytokines, biomarkers, multiplexes, and biological materials."
What scientific advancements are expected with the name change and knowledge growth?
"We've expanded our product offerings to include new assay technologies that the Quansys Q-plexTM, Forte Octet®, and Singulex Erenna® platforms encompass. And by growing the Product R&D and Assay Services group by several staff members this year, we have further bolstered our product development and assay service capabilities." Pestka added.
A new logo is now being seen at trade-shows, on business cards and on other promotional materials as Assay Science is the messaging to both existing and future customers. The goal is to grow and expand the company's reputation as a provider of high-level Assay Science to researchers around the world.
To learn more about how PBL Assay Science can help in solving your most difficult assay challenges, contact PBL Assay Science.
Official PR Newswire Press Release
Ultra-sensitive Cytokine assay services now available from PBL
June 1, 2013, Piscataway, NJ, USA --- PBL InterferonSource announces the expansion of its assay services portfolio to include 30 different ultra-sensitive biomolecule assays. Based on an innovative single-molecule counting (SMC) technology, these services are designed to provide sub-picogram per ml sensitivity for analytes in human serum or plasma. The background reduction methodology of this detection service extends the linear dynamic range to over 4 logs, allowing high levels of biomarker measurement with minimal dilution in many disease states.
"We are proud to add ultra-sensitive cytokine detection services into our offerings to researchers around the world." said Dr. Thomas Lavoie, Director of Product Research & Development and Assay Services at PBL. “The ability to measure basal levels of cytokines and other biomarkers in the femtogram per ml range will enable far deeper analysis of changes in these biomarkers. This enhanced understanding of various diseases and the effects of treatments will allow earlier project milestone decisions, saving our clients time, money and concern.”
The addition of ultra-sensitive cytokine detection services signifies PBL’s commitment in providing the best service solutions to meet scientists’ assay needs. It complements the company’s single-analyte ELISA, cytokine multiplex ELISA, and cell-based activity assay services that are currently offered in their sample testing and screening catalog.
Initial ultra-sensitive assay services by PBL include detection for cytokines such as human IL-17A, IL-17F, IL-17 A/F heterodimer, IL-6, IL-1a, IL-1b, IL-10, IL-15, TNF-a, GLP-1, and biomarkers such as cardiac troponin-I. For more information and a complete listing of biomolecules, contact PBL.
2012
PBL InterferonSource to collaborate with AVANZA Laboratories
January 9, 2012, Piscataway, NJ, USA --- PBL InterferonSource (PBL), a leading provider of interferon research tools and services, and AVANZA Laboratories, a preclinical CRO that provides high quality regulatory-compliant drug development services, announce a unique collaboration between their two organizations that will focus on enhancing their ability to service the biopharmaceutical industry.
The role and regulation of protein biomarkers in diseases is a major focus area, particularly in cancer, autoimmune disease and inflammatory disease. Understanding the role of biomarkers in various disease states is becoming increasingly important as drug development moves towards the use of biologics for therapeutics. The joint venture will enable the companies to provide a broader offering for their customers that will include specialized assay development, technology collaborations, and innovative platform applications which will support protein biomarker research and cytokine drug development.
PBL InterferonSource CEO Robert Pestka said: “We have a deep understanding of interferons and cytokines as therapeutics and potential biomarkers. It’s exciting to bring AVANZA’s complementary service offerings together with our technical expertise to serve the drug development industry.”
AVANZA Laboratories’ Executive Vice President Ira DuBey commented: “Scientists are just beginning to unravel the roles that interferons and cytokines have in various diseases. We believe our partnership with PBL will allow us to provide relevant data faster and increase the efficiency of drug development, saving time and money.”
Financial aspects of the collaboration were not released.
About PBL InterferonSource PBL InterferonSource, a division of Pestka Biomedical Laboratories, based in Piscataway (NJ, USA), is the world’s leading supplier of interferon products to the life science researcher. Founded in 1990 by Sidney Pestka, M.D., PBL is the company that scientists turn to for their interferon and cytokine-related needs: products, services and technical know-how. The company strives to aid researchers around the world in a common quest to help humanity.
About AVANZA Laboratories AVANZA Laboratories is a preclinical CRO that provides high quality regulatory-compliant drug development services. AVANZA is US owned and headquartered in Gaithersburg, Maryland. The AVANZA team is known for its extensive work in toxicology, including vaccines, developmental and reproductive toxicology (DART), safety pharmacology, product development support, and consulting services. Additional information about AVANZA Laboratories can be found at smithersavanza.com or by email at info-avanza@smithers.com.
2011
PBL InterferonSource introduces Quansys Q-Plex™ Cytokine Multiplex ELISA Arrays
New multiplexing tools for interferon detection help researchers better predict clinical outcomes
Piscataway, NJ – PBL InterferonSource, the world’s leading interferon expert for life science research, introduces the first Quansys Q-Plex Cytokine Multiplex ELISA arrays -- new high-throughput interferon detection kits that can save time and provide key information for drug discovery and clinical research efforts in the treatment of infectious diseases and autoimmunity. Developed by Quansys Biosciences, the kits are currently available exclusively through PBL InterferonSource internationally and to select U.S. institutions.
Q-Plex kits combine interferon reagents with multiplex arrays to give researchers a better understanding of the relationship between multiple interferons and cytokines. Examination of these relationships reveals key information that can help predict clinical outcomes based on several interferon-related biomarkers including:
- Assessment of cytokine profiles at various points throughout the course of disease
- Correlation of cytokine profile alterations with patient responses to therapies
- Determination of drugs’ off-target effects
The fully quantitative ELISA-based tests contain up to 16 distinct capture antibodies in each well of a 96-well plate. Each spot is well defined and represents a distinct antibody population. Up to 84 different samples can be assayed for all 16 unique cytokines in less than 2.5 hours. The ability to assess multiple cytokines allows researchers to spend more time interpreting data and less time accumulating data.
The current Q-Plex Multiplex ELISA products include six human and four mouse cytokine arrays that:
- Assay up to 16 cytokines in less than 2.5 hours
- Require less than 30 µl of sample
- Detect interferons from 30 pg/ml to less than 1 pg/ml
- Contain low percent assay-variability; intra-assay: 8% and inter-assay: 13%
- Compatible with serum, plasma, cell culture supernatant, homogenates, lysates, nasal lavage, tears and urine
Q-Plex Cytokine Multiplex ELISA arrays are just one of the high-performance products and assay services available from PBL InterferonSource for the life sciences community. In early 2010, PBL InterferonSource will introduce human interferon-based multiplex ELISA arrays that can simultaneously quantify the levels of Type I, II and III human interferons in a single sample. These human interferon-based kits are co-developed with Quansys Biosciences and will allow researchers to further explore complex interferon-related events.
To see what other interferon technologies are being distributed and/or developed at PBL InterferonSource, please contact us at info@pblassaysci.com.
PBL InterferonSource, a division of Pestka Biomedical Laboratories, Inc. based in Piscataway, NJ, is the world’s largest source of interferons and related products, assay services, and consulting to the life sciences research market.
Certain statements contained herein, including statements regarding development of the Company's products, services, markets, and future demands for the Company's products and services, and other statements regarding matters that are not historical facts, are forward-looking statements. Such forward-looking statements include risks and uncertainties; consequently, actual results may differ materially from those expressed or implied thereby.
PBL launches new Multiplex IFN/Cytokine ELISA kit
March 1, 2011, Piscataway, NJ – PBL InterferonSource, a leading provider of Interferon (IFN) research tools and services to life scientists, today announces the launch of the latest addition to their Multiplex ELISA line, VeriPlex Human Interferon Multiplex ELISA kit.
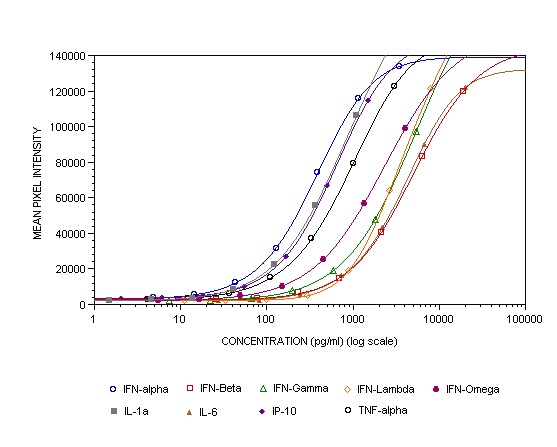

This new multiplex ELISA kit allows life scientists, for the first time, to simultaneously quantify Human Type 1, 2, and 3 interferons as well as related pro-inflammatory cytokines from a single biological sample.
"This new VeriPlex Human Interferon Multiplex ELISA kit emphasizes PBL’s commitment to provide high quality tools and services to help researchers answer key questions in Life Sciences" said PBL’s XXX. "By responding to researchers’ need to understand how different types of interferon are related to various disease states, and providing a solution in the simple and cost-effective multiplex ELISA platform* that can maximize the amount of information obtained from limited sample sources, we believe this kit will be a key tool to usher new scientific understandings in many interferon related immunological events."
VeriPlex Human Interferon Multiplex ELISA is just one of the highly accurate and reliable kits being developed every day at PBL InterferonSource for the life sciences community. To see what’s happening in the world of interferons, please contact us at info@pblassaysci.com.
* Powered by Quanysys Biosciences Q-Plex Multiplex ELISA technology
PBL InterferonSource, a division of Pestka Biomedical Laboratories, Inc. based in Piscataway, NJ, is the world’s largest manufacturer and distributor of interferons and related products to the life sciences research market.
Certain statements contained herein, including statements regarding development of the Company's products, services, markets, and future demands for the Company's products and services, and other statements regarding matters that are not historical facts, are forward-looking statements. Such forward-looking statements include risks and uncertainties; consequently, actual results may differ materially from those expressed or implied thereby.
2009
PBL InterferonSource introduces VeriKine-HS Human Interferon Beta Serum ELISA Kit
The accurate and reproducible way to measure low level Human Interferon-Beta
Piscataway, NJ – PBL InterferonSource introduces VeriKine-HS Human Interferon-Beta Serum ELISA Kit. The kit allows high sensitivity and accurate measurement of human Interferon-Beta (IFN-ß) level in different sample matrices.
IFN-ß is a tightly regulated, early response cytokine primarily responsible for controlling viral infection. Conversely, IFN-ß aberrant expression may be associated with inflammation and autoimmunity. More sensitive assays are needed to investigate the physiological relevance of low levels of IFN-ß that may be present before viral infection or autoimmunity is observable. However, a constant challenge in interferon immunoassays is to consistently quantify the amount of IFN-ß presence in serum and plasma without interference or high background caused by heterophilic antibodies, clotting factors and/or serum proteins in the samples
PBL InterferonSource’s latest offering, VeriKine-HS Human Interferon-Beta Serum ELISA kit, is designed to provide IFN researchers the ability to accurately and reproducibly measure the level of IFN-ß in human serum or plasma samples. It offers low serum/plasma background detection with dose response that is better than other ELISA kits currently available to scientists (Figure 1). It has superior sensitivity enabling researchers to determine IFN-ß level to as low as 0.5 IU/ml (Figure 2). It also has an excellent percent coefficient of variation (%CV) to ensure data reproducibility.
VeriKine-HS Human Interferon-Beta Serum ELISA is just one of the highly accurate and reliable kits being developed every day at PBL InterferonSource for the life sciences community. To see what’s happening in the world of interferons, please contact us at info@pblassaysci.com.
PBL InterferonSource, a division of Pestka Biomedical Laboratories, Inc. based in Piscataway, NJ, is the world’s largest manufacturer and distributor of interferons and related products to the life sciences research market.
Certain statements contained herein, including statements regarding development of the Company's products, services, markets, and future demands for the Company's products and services, and other statements regarding matters that are not historical facts, are forward-looking statements. Such forward-looking statements include risks and uncertainties; consequently, actual results may differ materially from those expressed or implied thereby.
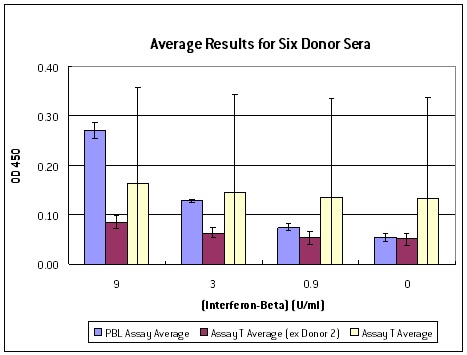

Figure 1: Interferon spike-recovery tests with six serum samples where known amount of IFN-ß was added to the sera. Columns in yellow represent the average results from another manufacturer’s kit with one false positive. Columns in red are the other kit’s average results minus the false positive. Average results from PBL’s VeriKine-HS Human IFN-ß Serum kit (blue) demonstrated a superior dose response than the other kit.
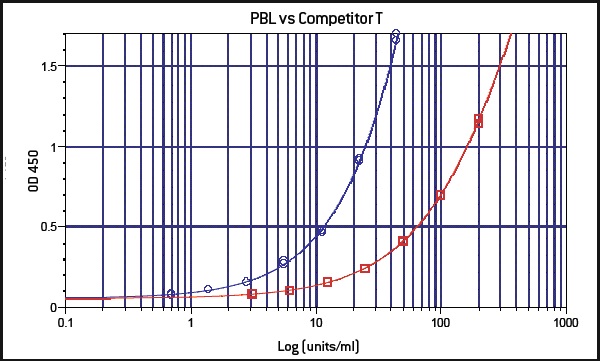

Figure 2: In a side by side comparison with another manufacturer’s kit (red), PBL’s VeriKine-HS Human Interferon-Beta Serum ELISA kit (blue) displays greater sensitivity.
